Cultivation of cells outside their natural habitat in a laboratory with controlled conditions is indispensable for scientific research. In particular, 3D cultures reflect in vivo conditions in cell morphology, interaction and tissue-specific architecture and are therefore relatively close to native tissue.
The disadvantage: Currently, 3D methods display a low experimental efficiency, have unclear standards and often poor reproducibility. Additionally, a thorough validation of these models prior to extensive application is necessary in contrast to standardized 2D-assays and animal models. Nevertheless, 3D models are the future. Knowledge about them is increasing on a daily basis and, due to advances in 3D printing (soon available: Corning Matribot Bioprinter) and technical platforms, it is going to be easier, faster and cheaper to build high-throughput platforms. But, what are 3D models currently capable of? The most commonly used 3D tissue models are called spheroids and organoids.
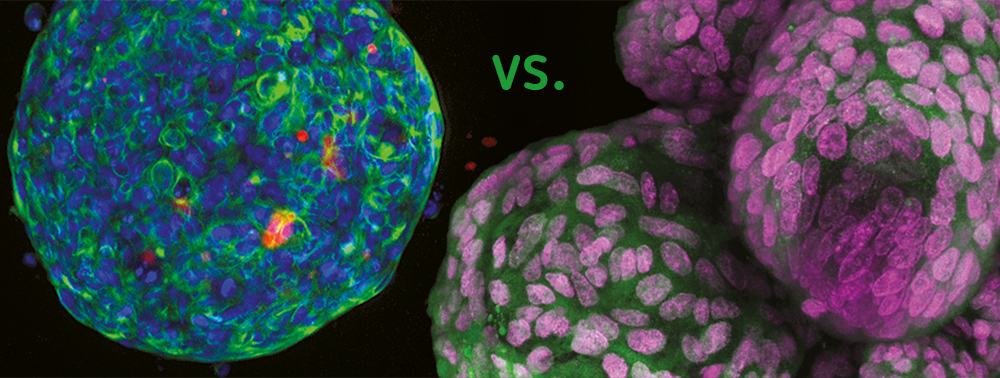
Spheroid – the first step

The term “spheroid” was first coined in the 1970s by R.M. Sutherland when he observed V79-lung cells from Chinese hamsters forming round-shaped cell aggregates in suspension in Spinner flasks. Since then, spheroids based on several primary cell types and cell lines have been manufactured with various methods, for instance with low cell attachment plates. Spheroids are frequently applied in cancer research since tumor cells form typical multicellular tumor spheroids (MCTS). They consist of an external proliferating region and an inner resting region, caused by a nutrition gradient and oxygen diffusion. The cell architecture within the MCTS simulates in vivo conditions regarding the cell morphology, proliferation, oxygenation, nutrition uptake, excretion of waste and drug absorption. Hence, the MCTS model is now one of the most distributed preclinical screening tools for new potential cancer medication.
Organoid – the step towards real tissue
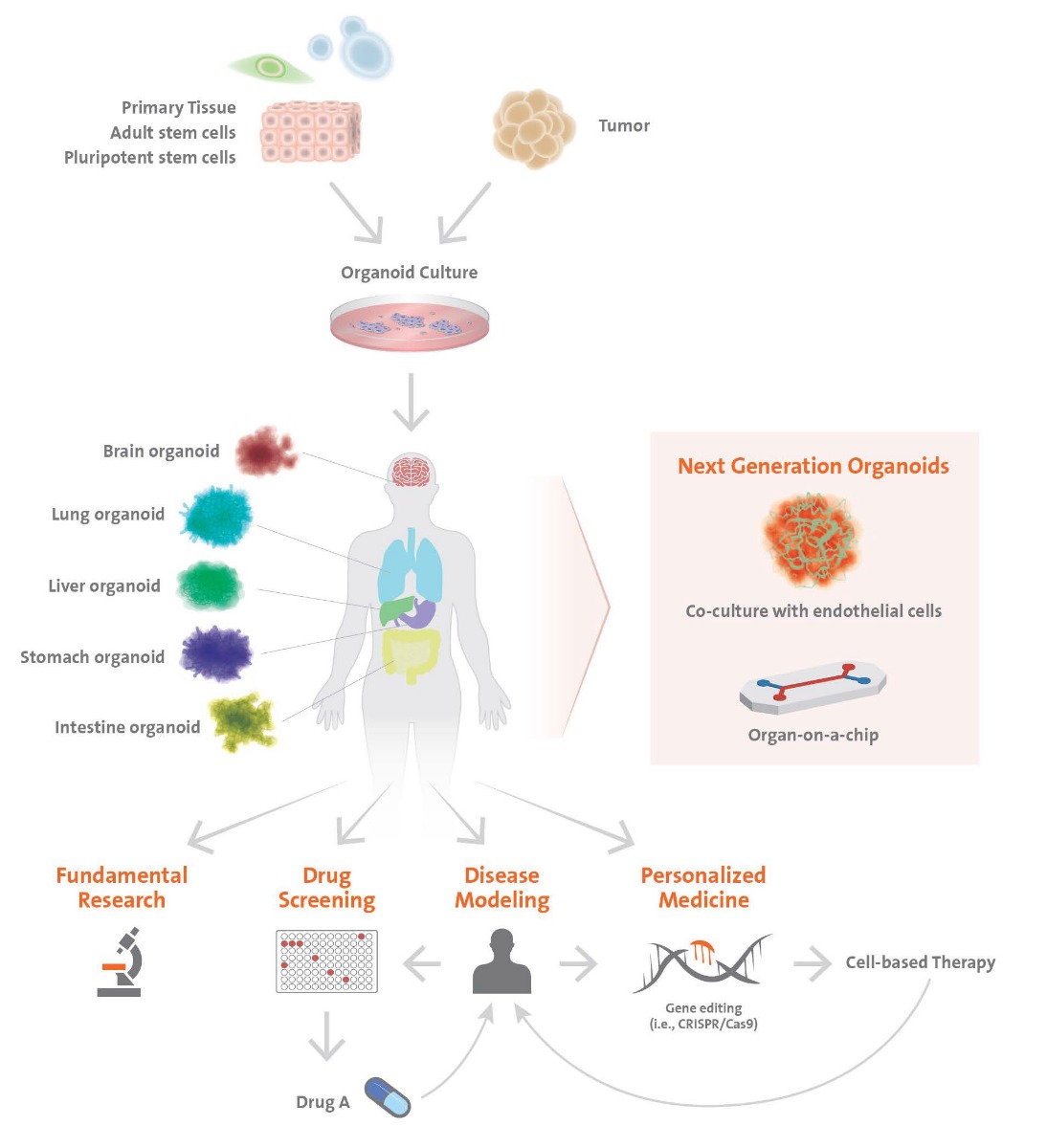
Organoid is a general term, describing 3D structures of stem cells or grown cell structures antecedent to organs. These consist of self-organized organ-specific cells, are spatially confined and have functions of tissues or organs.
Organoids can originate from adult tissue or pluripotent stem cells. Similar to in vivo organs, organoids contain several cell types, are organized in a structure similar to an organ and display some of the functionality of the organ. Models of the brain, liver, thymus gland, thyroids, lungs, pancreas, intestines and heart have already been established. These models are used, for instance, for detection or screening of drugs and toxicity tests. The interaction between the cells and the extracellular matrix (ECM) is essential for survival, proliferation, differentiation and migration. The ECM offers an environment in which the cells are able to move and grow three-dimensionally. Spheroid cultures are less dependent on matrices for their formation.
3D tissue models on chips
Even though 3D cultures, such as spheroids and organoids, are a distinctive improvement over 2D monolayer cultures, they are still not able to imitate the complete architecture of the tissues, including a proper vascular system and interstitial flow of liquids. The “organ on a chip” (OOC) technology is possibly going to be able to compensate some of the limitations. Here, cells are cultivated in continuously perfused, micrometer-sized chambers. During this process, physiologically relevant levels (=similar to in vivo tissues) of shear force, vascular nutrition, gas and waste transport are reached. The first kind of this system was called “lung on a chip”, developed by the Wyss Institute at Harvard University. With the chip, the effect of a bacterial infection on lung function was investigated. Since then, several model systems imitating the heart, kidney, arteries, bones, cartilage, skin and others have been developed.
OOCs and their similarity to native tissue allow them to be excellent tools to identify organ-specific, biomechanical and biochemical mechanisms and are found to be useful for drug screening. These platforms have the potential to lower costs, replace animal testing, and optimize drug research. Ultimately, the goal is a “human on a chip” system, where multiple organs are connected, to evaluate the response/reaction of the entire body and to replace cell cultures and animal tests. Hence, the “organ on a chip” technology is very promising. The path ahead, however, is a long one since native tissues are still too complex. Hence, more research in this area is still necessary.
Everything for 3D cell culture
Szabo-Scandic has been involved in the field of cell culture for several years now and offers all the consumables needed for a successful 3D cell culture – including cell lines and primary cells, as well as media and sera, petri dishes, flasks and microwell plates or buffers and markers and even cell counter and bioassays. The experience shows: patient and valid equipment lead to successful cell culture.
Further interesting articles:

 Deutsch
Deutsch




